421 Circular dichroism spectroscopy. 1 An example of a UV Vis absorption spectrum of Food Green 3 and a corresponding calibration curve using standard solutions are provided in Figure 5.

Ultraviolet Spectrophotometry An Overview Sciencedirect Topics

Negative Absorption Peaks In Ultraviolet Visible Spectrum Of Water Ji 2016 Chemistryselect Wiley Online Library

10 2 Spectroscopy Based On Absorption Chemistry Libretexts
Absorbance or spectral fingerprint the changes in concentration can be directly measured.

Uv spectroscopy negative absorbance. The amount of light absorbed is expressed as either transmittance or absorbance. In UV-Vis spectroscopy the wavelength corresponding to the maximum absorbance of the target substance is chosen for analysis. Absorbance is defined as the logarithm of the ratio of incident to transmitted radiant power through a sample excluding the effects on cell walls.
Rapidly characterize samples at different wavelengths and pathlengths using a single method. Detrimental the introduction of absorbance into IR spectroscopy. B CD spectra of.
Used the position of the plasmon band and the ratio between the absorbance at the plasmon band and the absorbance at 450 nm to estimate the mean diameter of gold NPs. Thus for routine UVVis spectroscopy the integrated absorbance can be used instead of the peak absorbance to establish calibration curves. Principles and applications of UV-visible spectroscopy Transmittance and absorbance When light passes through or is reflected from a sample the amount of light absorbed is the difference between the incident radiation Io and the transmitted radiation I.
Both NAD and NADH have strong UV absorbances but at 340 nm NADH has a much higher absorbance than NAD. Absorbance on the vertical axis is just a measure of the amount of light absorbed. UV-VIS Spectroscopy - Chemical Analysis Chemical Analysis Solutions Unit.
Electromagnetic Spectrum Type of Radiation Frequency Range Hz Wavelength Range Type of Transition Gamma-rays 1020-1024 Ultraviolet 1015-1017 400 nm-1 nm outer electron Visible 4-75x1014 750 nm-400 nm outer electron Near-infrared 1x1014-4x1014 25 mm-750 nm outer electron molecular vibrations. Contrariwise absorbance of visible light blue light by cis-azobenzene the nπ transition results in conversion back to the trans-isomer so does leaving cis-azobenzene in the dark a process known as thermal relaxation. This is the case for many of the dehydrogenase enzymes.
For this reason as shown in Figure 10210 it is better to make absorbance measurements at the top of a broad absorption peak. Circular Dichroism spectroscopy is widely used to assess HOS in biopharmaceuticals because the far-UV CD is sensitive to changes in the bond angles of a proteins backbone chain and secondary structure and the near-UV monitors the tertiary structure composed. The absorption or reflectance in the visible range directly affects the perceived color of the chemicals.
The resulting absorbance transformation would then be free from any artifactual data due to undefined absorbance from negative T numbers. Circular dichroism CD spectra of polypeptides and proteins with representative secondary structures. A CD spectra of poly-L-lysine at pH 111 in the 1 black α-helical and 2 red antiparallel β-sheet conformations and at pH 57 in the 3 green extended conformations 5 and placental collagen in its 4 blue native triple-helical and 5 cyan denatured forms 64.
Bonding configurations are readily predicted by valence-shell electron-pair repulsion theory commonly referred to as VSEPR in most introductory chemistry texts. Absorption of UV radiation is governed by the Beer-Lambert Law. A Ebc where A is the absorbance E is the molar absorptivity of the UV absorber b is the path length and c is the concentration.
Extinction spectroscopy is a noninvasive optical characterization technique which can be used to gain quantitative insights on NP morphology 19202122232425. 23 UV visible spectroscopy. The phenomenon of circular dichroism is very sensitive to the secondary structure of polypeptides and proteins Figure 21 and Figure 22 Circular dichroism CD spectroscopy is a form of light absorption spectroscopy that measures the difference in absorbance of right- and left.
Common examples include ultravioletvisible light absorption UVVis spectroscopy atomic absorption AA spectroscopy and Fourier transform infrared FTIR spectroscopy. This simple model is based on the fact that electrons repel each other and that it is reasonable to expect that the bonds and non-bonding valence electron pairs associated with a given atom will prefer to be as far apart as possible. The specific UV absorbance at 254 nm SUVA 254 was determined by dividing the UV absorbance measured at λ 254 nm by the DOC content of the sample Weishaar et al 2003.
You will see that absorption peaks at a value of 217 nm. For nonroutine use dispersion analysis can be used as an alternative which then allows not only the determination of the dispersion parameters but also the concentration of an analyte directly from the transmittance and reflectance spectra. This choice ensures maximum sensitivity because the largest response is obtained for a certain analyte concentration.
Absorbance of UV light by trans-azobenzene a ππ transition leads to isomerization to cis-azobenzene. UV spectroscopy or UVvisible spectrophotometry UVVis or UVVis refers to absorption spectroscopy or reflectance spectroscopy in part of the ultraviolet and the full adjacent visible regions of the electromagnetic spectrumThis means it uses light in the visible and adjacent ranges. The first and only slope spectrometer capable of Slope Spectroscopy-based measurements.
Transmittance be negative if negative values what. Figure 142 illustrates the principle of AA spectroscopy in which an atom absorbs the light of an incident photon exciting an electron from the ground energy state to a higher energy state. The higher the value the more of a particular wavelength is being absorbed.
Next Previous Up Top 42 Identification without 3D Structure. On-site installation and training included with. Some materials eg glycerin will have a slight negative absorbance because they contain trace amounts of fluorescent impurities you can purchase spectroscopy grades of many materials though.
The diagram below shows a simple UV-visible absorption spectrum for buta-13-diene - a molecule we will talk more about later. Since its frequency is close to the overtone frequency of many natural vibrations weak substance-specific absorption bands can be detected. Stray light causes a negative bias in instrument response and eventually is the limiting factor for the absorbance and thereby concentration that can be measured GroupPresentation Title.
Alternatively for samples which scatter light absorbance may be defined as the negative logarithm of one minus absorptance as measured on a uniform sample. A double beam systronics UV-visible spectrophotometer model UV. The ratio of absorbance at 250 nm to 365 nm was calculated to obtain E 2 E 3 Peuravuori and Pihlaja 1997.
Because the absorbance is directly dependent on the path length UV absorbers provide little or no protection at the surface of a part where the path length is very short. From UV machinekindly help to find absorbance and transmittance. A Absorbance at 517nm Blank 0004 DPPH reagent The percentage of the standard and sample was compared by plotting graph of antioxidant concentration against free radical scavenging activity.
Polychromatic radiation always gives a negative deviation from Beers law but the effect is smaller if the value of varepsilon essentially is constant over the wavelength range passed by the wavelength selector. The term is used in many technical areas to quantify the results of an experimental. UV-VisibleNIR spectroscopyUV-Vis Spectroscopy can be divided into ultraviolet visible and near-infrared regions of the spectrum depending on the wavelengths used.
Since the correct minimal level of smoothing is basically a trial and error procedure this type of methodology is difficult to automate properly in instrument software. Slope results based upon multiple data points instead of a single absorbance value.
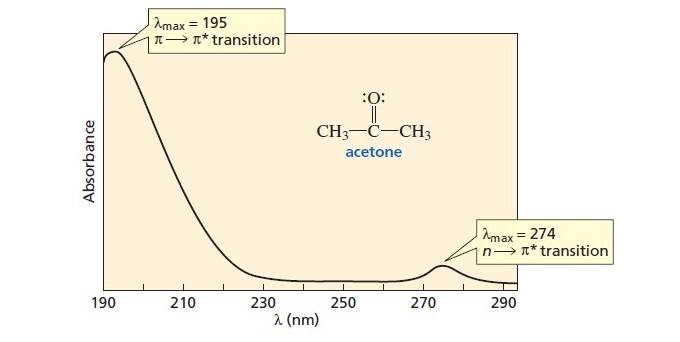
Uv Vis Spectroscopy Absorbance Of Carbonyls Master Organic Chemistry
Biochromspectros Com

Determination Of Ultra Low Milk Fat Content Using Dual Wavelength Ultraviolet Spectroscopy Sciencedirect
1
1
Why The Absorbance Peak Is At 540 Nm For Nacl Solution Prepared In Distilled Water

A Review On Derivative Uv Spectrophotometry Analysis Of Drugs In Pharmaceutical Formulations And Biological Samples Review

What Does A Negative Absorbance Mean On Uv Vis And Why Am I Getting It

